Alloy Self-Protection
What is alloy self-protection?
In short – the thermal oxides formed after Minimox® treatment have:
1. Better surface chemistry, and
 |
UntreatedTreated | Surface Cr/Fe ratio improvement Bulk 410 Cr/Fe ~ 0.14 Untreated & oxidized 410, Cr/Fe = 0.03 Treated & oxidized 410, Cr/Fe = 5.7 The surface oxide after Minimox® treatment and suitable oxidation is Cr-Mn-O spinel, even though there is no Cr or Mn in the solution. |
2. Better surface structure
[twocol_one]
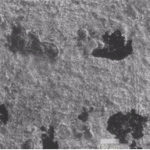
Uncoated 600
[/twocol_one]
[twocol_one_last]
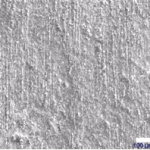
Coated 600
[/twocol_one_last]
The composition of the protective layer is primarily a function of the alloy, not the coating material.
The Minimox® Process
Alloys designed for high-temperature applications contain certain additions. Research has shown if those addition elements are instead applied as a superficial self-protective alloy treatment to the surface of a more basic alloy, the same high-temperature surface oxidation properties can be achieved.
The nanoparticles are in liquid suspension. The patent-pending Minimox® process is not a coating, per se, but is more accurately described as a “surface treatment” or “surface doping.” The solution contains nanoparticles that are superficially applied to stainless steels, nickel alloys, superalloys and aluminum alloys. The nanoparticles cause resulting thermal oxides to have a different chemistry and structure when compared to untreated materials. The thermal oxides are significantly thinner, but more impervious. After oxidation, the treatment causes the alloy to become more “self-protective.” The composition of the ultimate protective coating is primarily a function of the alloy, not the coating material.Minimox® self-protective alloy treatment solution provides well-dispersed, extremely small particles required for a superior coating with astonishing protection. After self-protective alloy treatment, alloys heated in air exhibit a thin, adherent, dense oxide layer, rather than a thick, flaking, spalling oxide scale. The coating is applied through dipping, brushing, or spraying followed by air drying. If there is oxygen present during service, there is no other processing necessary. If there is little oxygen present during service, the surface will need to be preoxidized.
Development is in progress for many applications and alloys. Results detailed in this website are a combination of laboratory experiments and industrial trials. Your specific alloy, environment, and conditions will influence your results. Minimox® self-protective alloy treatment is for experimental use only and no guarantees can be made for specific outcomes. Contact us with your requirements.
